Abstract

Introduction: Venetoclax (Ven) is a selective, potent, oral BCL-2 inhibitor under investigation as a targeted therapy for t(11;14)-positive relapsed/refractory multiple myeloma (RRMM). The molecular mechanisms underlying the increased sensitivity of t(11;14) MM to Ven have not been fully elucidated; however, remnants of B-cell biology and reduced cellular bioenergetics have been associated with sensitivity to Ven in MM. Transcriptomic analysis of 302 RRMM patient tumor cells before treatment with Ven-based regimens, an ex vivo B-cell differentiation model, and metabolic profiling of 7 MM cell lines were assessed to understand the unique biology that distinguishes t(11;14) in MM.
Methods: CD138-enriched bone marrow mononuclear cells were collected at baseline from multiple Ven clinical trials (NCT02755597, NCT01794520, NCT03314181, NCT02899052). Biomarker analyses, including BCL2 gene expression by quantitative PCR, t(11;14) status by interphase fluorescence in situ hybridization (FISH), and transcriptomic analysis by RNA sequencing (RNA-seq), were performed by a central laboratory. Isolated healthy memory B-cells (MBCs) were progressively differentiated ex vivo into pre-plasmablast (Pre-PB), plasmablast (PB), and plasma cells (PC). MM cell lines were differentiated by treatment with all-trans retinoic acid (ATRA) for 3-days. Global metabolomic profiling was performed by liquid chromatography-mass spectrometry (LC-MS) in MM cell lines with or without ATRA-mediated differentiation. Gene set variation analysis (GSVA) was used to establish B-cell and mitochondrial (Mito) gene signatures.
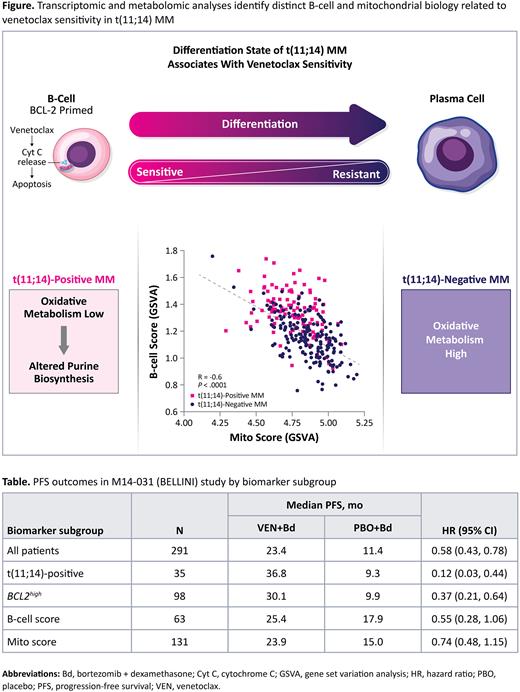
Results: Consistent with previous findings, t(11;14)-positive RRMM expressed significantly higher CCND1 (mean log2 FPKM: 3.8 vs −0.4, P<.0001) and BCL2 levels (mean 2-ΔCt: 1.3 vs 0.4, P<.0001) compared with t(11;14)-negative RRMM. Using a network-based enrichment map to interrogate differentially expressed genes, transcriptomes of t(11;14)-positive samples were enriched in several pathways, including cell cycle, metabolism and biosynthesis, and immune pathways. Significantly higher B-cell signature scores, but lower Mito signature scores were detected in t(11;14)-positive versus t(11;14)-negative RRMM patients (P<.0001; Figure). The ex vivo B-cell differentiation model confirmed that B-cell and Mito scores are differentiation-stage specific and significantly changed upon differentiation to plasma cells (P<.0001). In RRMM patients, there was an inverse correlation between the B-cell and Mito signature scores (R=−0.6, P<.0001). In vitro models were then used to further investigate the relationship between B-cell differentiation and Ven sensitivity. ATRA differentiated t(11;14)-positive MM cell lines, as indicated by lower expression of CD20 and increased expression of the plasma cell markers CD138 and CD38, significantly reduced BCL2 expression and increased basal rates of respiration in association with increased mitochondrial mass, which correlated with reduced Ven sensitivity. Differentially expressed metabolites were overrepresented in the purine biosynthetic pathway in t(11;14)-positive MM cell lines, which was corroborated by differential gene expression within this pathway in the ex vivo B-cell differentiation model and in t(11;14)-positive RRMM patients. Using thresholds that balance sensitivity and specificity of the B-cell and Mito signature scores associated with t(11;14)-positive RRMM, progression-free survival (PFS) outcomes in the BELLINI study were not superior to those demonstrated by t(11;14) or high BCL2 expression status alone (Table).
Conclusions: Using an integrated approach of transcriptomic and metabolomic profiling, t(11;14) MM cells were confirmed to retain features of B-cell biology, including lower Mito metabolism that results in increased sensitivity to BCL-2 inhibition by Ven. Although these biological features are closely linked to t(11;14), B-cell gene or Mito gene signatures did not serve as better biomarkers for predicting efficacy to Ven compared with t(11;14) alone.
Disclosures
Sharon:Abbvie: Current Employment, Current equity holder in publicly-traded company, Other: TRAVEL, ACCOMMODATIONS, EXPENSES. Dunbar:Abbvie: Current Employment, Current equity holder in publicly-traded company. Jung:Abbvie: Current Employment, Current equity holder in publicly-traded company. Wang:Abbvie: Current Employment, Current equity holder in publicly-traded company. Li:Abbvie: Current Employment, Current equity holder in publicly-traded company. Mantis:Abbvie: Current Employment, Current equity holder in publicly-traded company. Bueno:Abbvie: Current Employment, Current equity holder in publicly-traded company. Moreau:AbbVie, Janssen, Celgene, Amgen, and Sanofi: Honoraria. Harrison:Abbvie, Amgen, Celgene/BMS, GSK, Janssen Cilag, Novartis, Roche Genentech, Eusa: Speakers Bureau; Abbvie, Amgen, Celgene/BMS, GSK, Janssen Cilag, Novartis, Roche Genentech, Haemalogix, Eusa, Terumo BCT: Honoraria; Celgene/BMS, GSK, Janssen Cilag, Haemalogix: Research Funding; Abbvie, Amgen, Celgene/BMS, GSK, Janssen Cilag, Novartis, Roche Genentech, Haemalogix, Eusa, Terumo BCT: Consultancy; Haemalogix: Membership on an entity's Board of Directors or advisory committees. Costa:Bristol Myers Squibb: Consultancy, Honoraria, Research Funding; Adaptive Biotechnologies: Consultancy, Honoraria; AbbVie: Research Funding; Genentech: Research Funding; Sanofi: Consultancy, Honoraria; Amgen: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Research Funding. Kaufman:AbbVie, Genentech, and Bristol Myers Squibb: Consultancy; AbbVie: Other: Member of steering committee; Incyte: Other: Member of data safety monitoring committee. Bahlis:AbbVie, Amgen, Bristol Myers Squibb, Celgene, Forus, Janssen, Genentech, GSK, Karyopharm, Novartis, Pfizer, Takeda, Sanofi: Consultancy; Pfizer: Research Funding. Kumar:AbbVie,: Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen,: Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda,: Membership on an entity's Board of Directors or advisory committees, Research Funding; Adaptive,: Membership on an entity's Board of Directors or advisory committees, Research Funding; KITE,: Research Funding; MedImmune/Astra Zeneca,: Membership on an entity's Board of Directors or advisory committees, Research Funding; Merck,: Research Funding; Novartis,: Research Funding; Roche: Research Funding; Sanofi: Research Funding; Oncopeptides: Other: Independent review committee. Ross:AbbVie: Current Employment, Current equity holder in publicly-traded company. Epling-Burnette:Abbvie: Current Employment, Current equity holder in publicly-traded company, Other: Travel Accommodations; Merck, Teva, and AstraZeneca: Current equity holder in publicly-traded company.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal